Our research aims to understand the mechanisms that drive Alzheimer’s disease and primary tauopathies progression, with the key goal of predicting and tracking disease trajectories at the individual patient level. We focus on how tau and amyloid pathologies interact with large-scale brain networks, how genetic, inflammatory and vascular factors shape these processes, and how these mechanisms translate into neurodegeneration and cognitive decline. To achieve this, we integrate multi-modal neuroimaging (PET and MRI) with fluid biomarkers, genetics, and clinical data from large, longitudinal patient cohorts. By combining mechanistic insight with clinical translation, our work advances precision biomarkers that improve prognostication, monitoring, and therapeutic trial design in Alzheimer’s disease and related tauopathies.
Our research can be broadly divided into five main topics:
1. Understanding How Brain Networks Drive the Spread of Tau
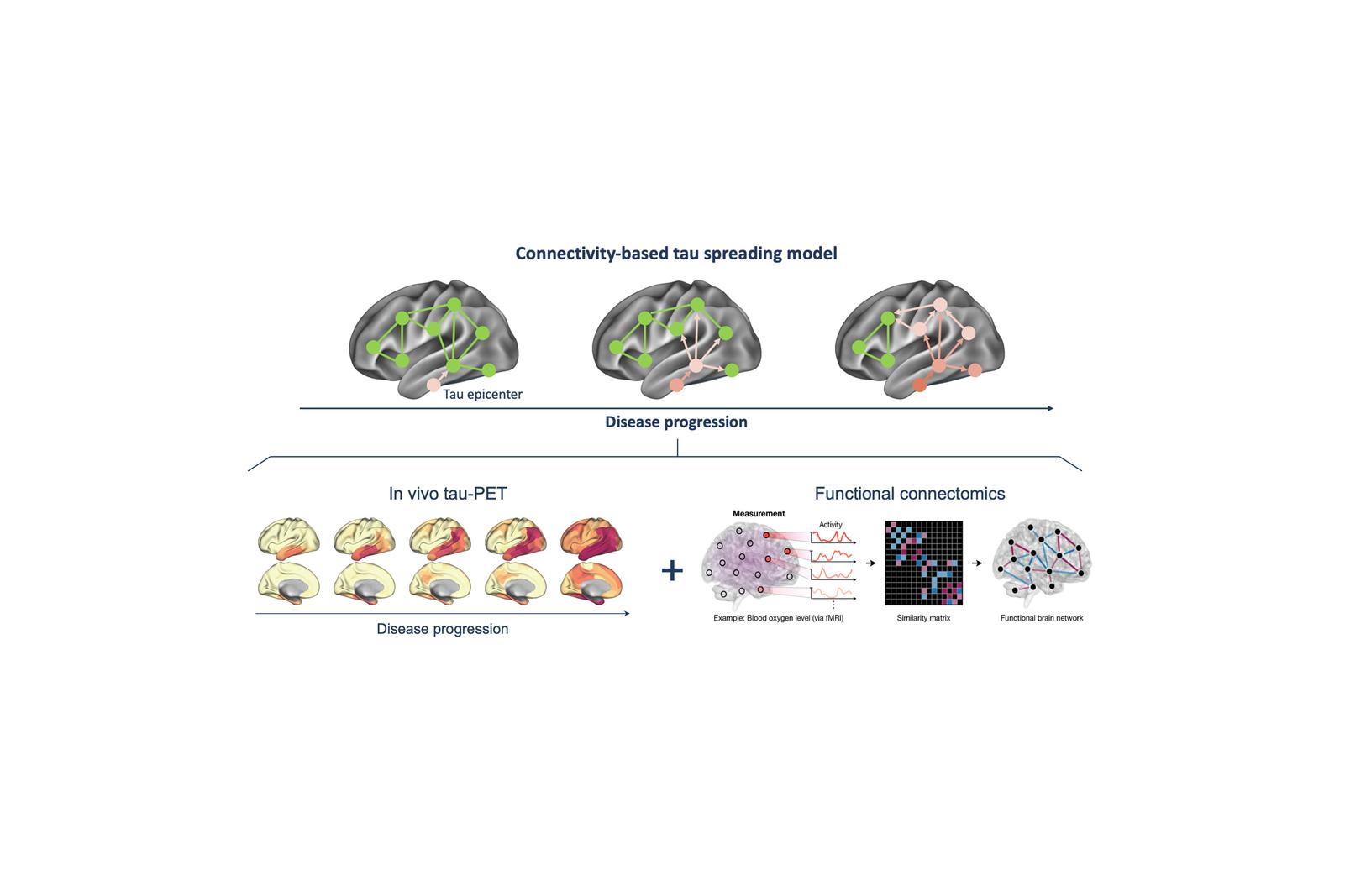
A central focus of our research is understanding how tau pathology propagates through the brain in Alzheimer’s disease patients. Rather than spreading randomly, tau spreads trans-synaptically, following the brain’s structural and functional connections. By combining tau-PET, MRI, and network analysis, we show that tau accumulates preferentially in regions strongly connected to already affected areas, i.e., so called tau epicenters. This network-based perspective not only provides a mechanistic explanation for the stereotypical progression of Alzheimer’s disease but also enables us to predict where tau will emerge next to determine patient-centered readouts in tau targeting trials. Our work highlights the brain’s connectome architecture as both a vulnerability factor and a potential therapeutic target.
Core team: Sebastian N. Roemer-Cassiano, Fabian Hirsch, Hannah De Bruin
Key publications:
2. Uncovering How Amyloid, Genetic Factors and Co-Pathologies Accelerate Tau Pathology
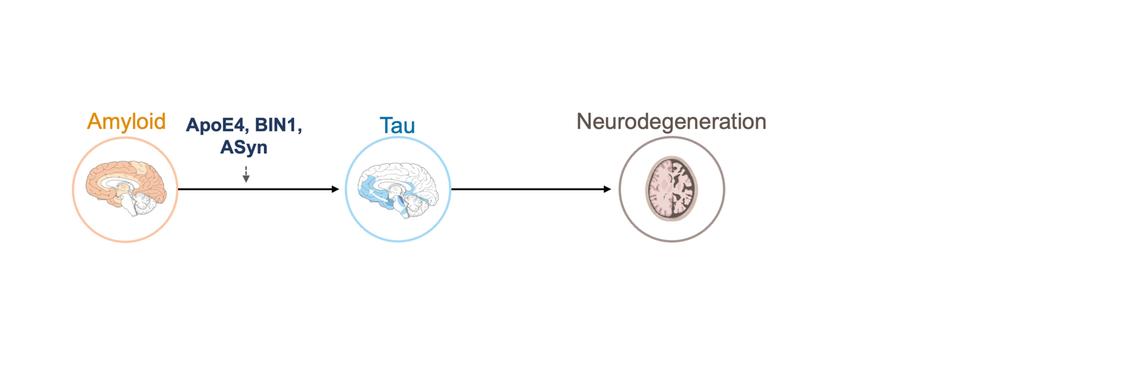
We study how amyloid pathology accelerates tau accumulation and spread, and why this process differs between individuals. Our research shows that amyloid promotes synaptic abnormalities and network hyperconnectivity, which in turn drive the spread of tau across brain networks. In addition, genetic and molecular factors such as APOE, BIN1, and the presence of tau-synergistic co-pathologies (e.g., α-synuclein) critically modulate these effects. To dissect these mechanisms, we integrate amyloid- and tau-PET, genetics, and fluid biomarkers across large cohorts. This line of work is motivated by the need to understand why some patients progress rapidly while others remain stable, paving the way for precision medicine approaches that tailor risk assessment and interventions.
Core team: Anna Steward, Madleen Klonowski, Zeyu Zhu
Key publications:
3. Developing Biomarker applications to Predict and Monitor Disease Progression
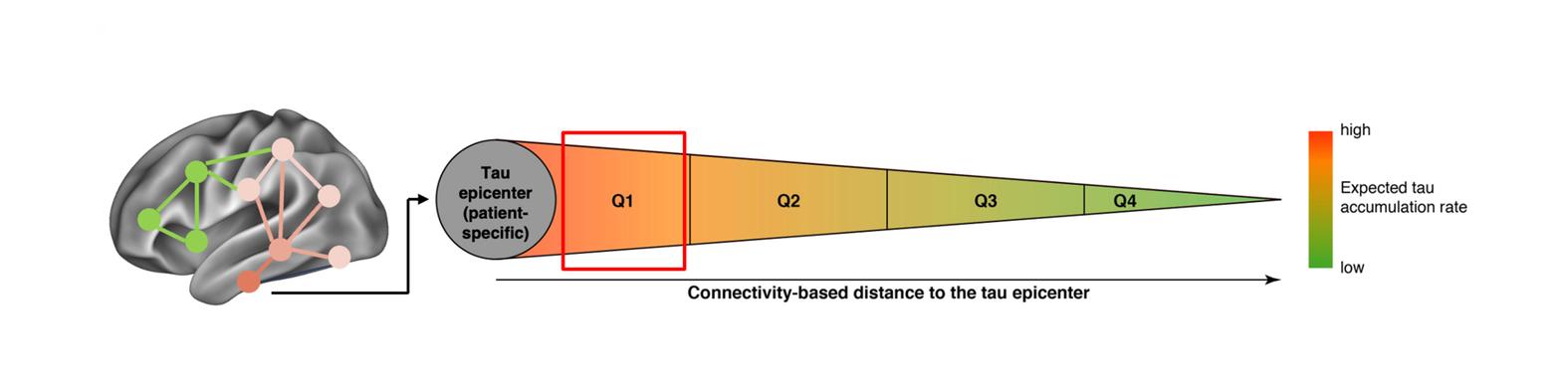
Our mechanistic work translates directly into clinical applications. We develop and validate multi-modal biomarker workflows that combine advanced imaging and fluid markers to enable earlier and more accurate diagnosis of Alzheimer’s disease and related tauopathies. These approaches improve the ability to predict individual disease trajectories and provide patient-tailored readouts to monitor therapeutic response over time. Importantly, our biomarkers are designed to support differential diagnosis, helping to distinguish Alzheimer’s disease from related tauopathies and other neurodegenerative conditions. By providing precise, biologically grounded measures of disease stage and progression, our work can directly inform patient stratification in clinical trials and enhance the evaluation of disease-modifying therapies via patient-tailored endpoints. Ultimately, we aim to translate these advances into routine clinical practice, where they can empower clinicians with reliable tools for personalized prognostication, diagnosis, and treatment monitoring.
Core team: Davina Biel, Zeyu Zhu, Anna Dewenter
Key publications:
4. Extending Tau Research Beyond Alzheimer’s to Primary Tauopathies
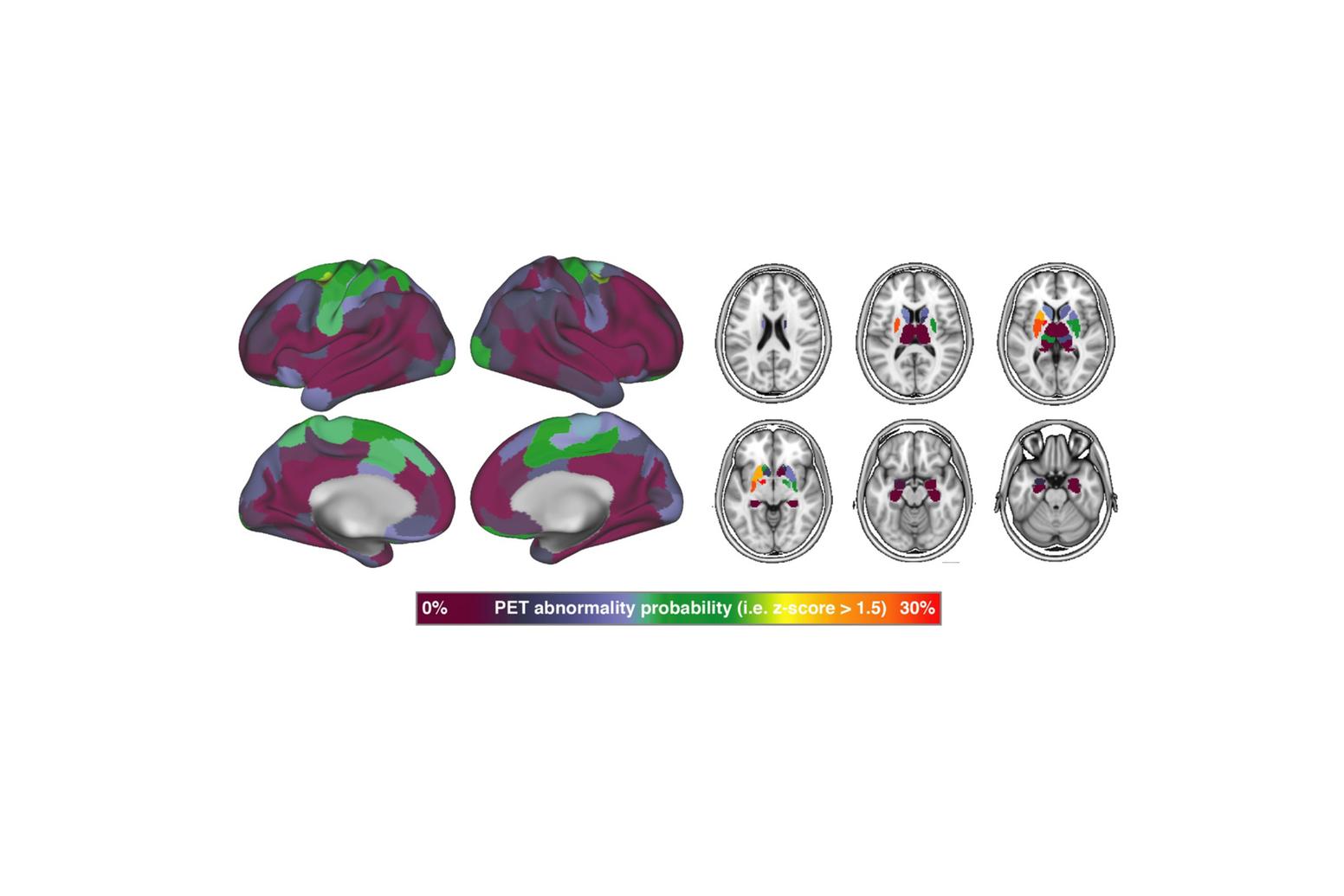
In addition to Alzheimer’s disease, our group investigates primary tauopathies such as progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), in close collaboration with the LMU Department of Nuclear Medicine and Department of Neurology. These disorders share tau pathology as their defining feature but present with distinct clinical and imaging phenotypes. Using the 2nd generation tau-PET tracer PI-2620, structural and functional MRI, and fluid biomarkers, we map tau distribution and the resulting network disintegration in these conditions. This research provides crucial insights into the heterogeneity of tau biology across diseases, supports the development of diagnostic biomarkers for PSP and CBD, and helps to define shared and distinct mechanisms of tau-driven neurodegeneration.
Core team: Lukas Frontzkowski, Sebastian N. Roemer-Cassiano
Key publications:
5. Understanding How Vascular and Inflammatory Brain Changes modify Alzheimer’s Disease Progression
Another line of our research focuses on the role of vascular and inflammatory brain changes in Alzheimer’s disease. By combining PET imaging, inflammatory fluid biomarkers, and advanced MRI measures of vascular brain health, we investigate how vascular and inflammatory processes interact with amyloid and tau pathology to drive neurodegeneration and cognitive decline. This work provides key insights into the synergistic interplay of vascular, inflammatory, and protein pathologies in Alzheimer’s disease and related tauopathies, underscoring the importance of considering vascular integrity and neuroinflammation in both mechanistic disease models and the development of therapeutic strategies.
Core team: Anna Dewenter, Davina Biel, Melina Paulus
Key publications:
Key collaborators
LMU Department of Nuclear Medicine: Matthias Brendel, Johannes Gnörich
LMU Department of Neurology: Günter Höglinger, Carla Palleis, Johannes Levin
Alzheimer Center, Vrije Universiteit Amsterdam: Rik Ossenkoppele, Colin Groot
University of Gothenburg: Michael Schöll
University of Cambridge: Maura Malpetti
Lund University: Oskar Hansson, Jacob Vogel
Barcelonaeta Brain Research Center: Gemma Salvadó
VASCage-Centre on Clinical Stroke Research Innsbruck: Annemieke ter Telgte
Core facilities
Image processing hub: At the ISD, we operate a dedicated high-performance computing (HPC) hub for large-scale multimodal image processing. Using our in-house developed ADprep pipeline, we have the capacity for large-scale processing of MRI and PET scans across multiple PET tracers (amyloid, tau, FDG, TSPO) and MRI modalities (structural, functional, diffusion). The hub enables standardized, automated workflows for structural and functional MRI as well as multi-tracer PET (amyloid, tau, FDG, TSPO), ensuring harmonized and reproducible outputs across studies and cohorts. Leveraging our HPC cluster, we provide image processing support for key collaborators at LMU Munich and international research partners, facilitating large-scale neuroimaging analyses and collaborative data sharing.

ICON (Individual Connectome Profiles In Alzheimer’s Disease):
PIs: Nicolai Franzmeier & Matthias Brendel
The ICON (Individual Connectome Profiles in Alzheimer’s Disease) cohort is a prospective longitudinal deep-phenotyping study designed to uncover how amyloid pathology, functional brain connectivity, and tau deposition interact in driving Alzheimer’s disease progression. The study includes amyloid-positive individuals across the Alzheimer’s disease spectrum, who undergo extensive multimodal imaging and biomarker assessments. ICON uniquely combines ~30 minutes of high-resolution resting-state fMRI for precision mapping of subject-specific connectivity with amyloid- and second-generation tau-PET, as well as high-resolution structural and diffusion MRI. By integrating these data with clinical and fluid, ICON will allow us to test whether amyloid-associated hyperconnectivity promotes tau spreading and subsequent neurodegeneration. This cohort will provide a powerful resource for understanding the mechanisms of Alzheimer’s progression and for developing predictive, patient-tailored biomarkers. Recruitment is ongoing at the outpatient clinic of the Institute for Stroke and Dementia Research.
For patients: https://www.lmu-klinikum.de/isd/klinische-studien-am-isd/icon-studie-nbsp/fb6df6c9d794b1ff
Study physicians: Katharina Bürger, Daniel Janowitz
Study neuropsychologists: Michaela Müller, Julia Friedl
Study nurses: Adelgunde Zollver, Brigitte Faschinger

Nicolai Franzmeier, Principal Investigator
PI data sheet:
Nicolai Franzmeier, PhD
Date of birth: 05 April 1989 in Munich
Junior Research Group Leader
Institute for Stroke and Dementia Research
Ludwig-Maximilians-University (LMU), Munich, Germany
And
Research Associate
Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Mölndal and Gothenburg, Sweden
Scientific vita:
Since 01/2023: Research Associate, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden
Since 01/2022: Associated Investigator, Munich Cluster for Systems Neurology, SyNergy, LMU University Hospital, Munich
Since 2021: Junior Research Group Leader, Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich
2017-2020: Post-doctoral researcher, Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich
2014-2017: PhD, Graduate School for Systemic Neurosciences, Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich
Coordinating functions, honors and awards:
2024 Alzheimer’s Association DeLeon Award
2022 Rudi Klose Alzheimer Research Award
2022: Helga Freyberg Rüßmann Award for Medical Research
2020: Bayer Early Excellence in Science Award
2019-2021: Vice-Chair: Reserve, Resilience and Protective Factors Professional Interest Area (PIA), ISTAART, Alzheimer’s Association
2017-2019: Programs Chair: Reserve, Resilience and Protective Factors Professional Interest Area (PIA), ISTAART, Alzheimer’s Association
2017: Steinberg-Krupp Alzheimer Research Award


Nicolai Franzmeier, PhD, Principal Investigator
I am an early career investigator with a strong focus on Alzheimer’s disease neuroimaging research. I received undergraduate training in Psychology and Medicine in Innsbruck, Austria, after which I completed my PhD at the graduate school for systemic neurosciences (LMU) in Munich. I am specifically interested in the mechanisms that drive the progression of Alzheimer’s disease and primary tauopathies. My overall goal is to develop clinically useful models for predicting disease progression and to identify therapeutically relevant targets for secondary prevention of Alzheimer’s disease and primary tauopathies. To this end, I am combining multimodal MRI and PET imaging with fluid biomarkers and genetics.

Davina Biel, PhD, Postdoc
I received undergraduate training in Psychology and Neurosciences from the University of Bremen, including lab rotations in Barcelona and Melbourne. Afterwards I moved to Lübeck to start my PhD in Neuroscience, focusing cognitive training effects and structural brain changes in older adults. I graduated in September 2020 and since then join the ISD as a post-doc in Munich focusing on neuroimaging in Alzheimer’s disease. I am interested in mechanisms of tau-spreading, genetics, and inflammatory processes which help to understand the progression in Alzheimer’s disease and facilitate patient-specific risk prediction. Outside the lab, I like to spend my time hiking, practicing yoga, and delving into classic literature.

Anna Dewenter, PhD, Postdoc
I obtained my Bachelor’s degree in Cognitive Science from the University of Osnabrück, including a study semester at the University of York (UK). I then completed a Research Master’s in Cognitive Neuroscience at the Donders Institute for Brain, Cognition, and Behavior (Nijmegen, Netherlands). Subsequently, I joined the ISD and the Graduate School for Systemic Neurosciences as a PhD student, where my research focused on structural connectivity alterations in cerebral small vessel disease (SVD) and Alzheimer’s disease. I successfully defended my PhD in December 2022. Since then, I have been working as a Postdoctoral researcher, with a focus on Cerebral Amyloid Angiopathy (CAA) – the most common intersection of cerebrovascular and neurodegenerative diseases. Outside of research, I enjoy hiking in the nearby Alps and traveling.

Sebastian N. Roemer-Cassiano, MD, Clinician Scientist
After receiving my Bachelor‘s degree in Human and Molecular Biology at the University of Freiburg and Saarland, I went on studying Medicine at the University of Saarland and Ludwig-Maximilians University Munich (LMU). I conducted my doctoral thesis at the university clinic of the Technical University Munich (TUM, Neuroimaging department). I am currently doing my residency in the department of neurology at the university hospital Munich (LMU, Neurology) as well as my PhD with the Max Planck School of Cognition. In August 2021 I joined the lab of Nicolai Franzmeier at the ISD, trying to shed light on the pathophysiology of Alzheimer’s disease and primary tauopathies, using advanced, state-of-the-art neuroimaging methods. Outside of the lab I enjoy meeting friends, travelling, reading and playing chess.

Lukas Frontzkowski, MD, Clinician Scientist
After finishing my study of medicine in 2021 at the LMU, I worked as a clinician scientist in the department of neurology at the UKE Hamburg. My main research focus is 1) biomarker-driven precision medicine and 2) neuroprotective mechanisms in tauopathies and stroke. Building upon my prior experiences in neuroimaging, I joined the Franzmeier lab in 2024 to establish advanced image analysis pipelines for PET data to enable high throughput imaging in clinical settings. In my free time I enjoy going to the gym, reading and going out with friends.

Anna Steward, PhD, Postdoc
I am a postdoctoral researcher with a background in psychology (BSc) and cognitive neuroscience (MSc). During my PhD at LMU Munich, I investigated modulators of tau spreading in Alzheimer’s disease, showing how functional network topology and the genetic risk factor APOE4 influence disease progression. As a postdoc, I focus on APOE4-specific mechanisms, aiming to define biomarker signatures in fluid and neuroimaging data that capture critical phase transitions in the disease. Outside the lab, I love hiking and skiing in the Bavarian alps and visiting the many Volksfeste here in Munich.

Fabian Hirsch, PhD, Postdoc
I am a psychologist and obtained my PhD in 2025 from the Technical University of Munich (Department of Neuroradiology), where I focused on connectomics in healthy and clinical populations. In the fall of 2024, I joined the lab of Nico Franzmeier at LMU, where I am currently supported by the MCSP scholarship of the LMU medical faculty. My research investigates how features of brain networks shape disease progression in Alzheimer’s disease. In parallel with my PhD, I worked part-time as a neuropsychologist at the ISD in-patient clinic, where I remain a certified rater for clinical trials. Outside of the lab I try to avoid visiting the many Volksfeste here in Munich.

Hannah De Bruin, PhD student
I obtained a Bachelor’s degree in Psychology at the VU Amsterdam, with a specialization in Biological- and Neuropsychology. Within this program, I got the opportunity to spend a semester at the University at Buffalo in New York State (USA). While taking several neuroscience courses, I developed a strong interest in the complex mechanisms of the human brain and went on to obtain both a two-year Research Master in Neurosciences in Amsterdam as well as a clinical Master’s degree in Neuropsychology. After graduation, I worked as a neuropsychologist in a nursing home for a year, and in June of 2022 I started my PhD in a cross-border research project in the labs of Nicolai Franzmeier at the ISD and in Rik Ossenkoppele’s lab at the Alzheimer Center Amsterdam. In my PhD-project, we explore functional connectivity as a predictor of tau spreading in atypical variants of Alzheimer’s disease by combining PET and post-mortem data. I am doing my PhD part-time, as I carry on with my work as a neuropsychologist for one day a week. In my leisure time, I like to meet with friends, watch movies, go swimming, travel, and cuddle with my cats.

Zeyu Zhu, PhD student
After receiving a Bachelor's degree in Clinical Medicine I went on to achieve a Master's degree in Neurology from Shanghai Jiao Tong University, China. In the autumn of 2023, I joined the Franzmeier lab to pursue my PhD. Currently, my research is focused on characterizing how tau accumulates and spreads in Alzheimer’s disease by combining neuroimaging, omics, and disease-progression models. Outside the lab, I love traveling and cooking, and I'm also a big fan of photography.

Madleen Klonowski, PhD student
I received a Bachelor’s and Master’s Degree in Speech and Language Therapy at LMU Munich with a specialization in neurology. After gaining a few years of clinical experience as a therapist in neurological rehabilitation, I went on to obtain an MSc in Interdisciplinary Neuroscience at the University of Frankfurt am Main, including a lab rotation at the University of Bern. I joined Nico Franzmeier’s lab in autumn 2024 for my Master’s thesis under the supervision of Sebastian Römer-Cassiano, where I leveraged MRI and PET data to explore associations between amyloid-beta, neuronal hyperactivity and tau spreading in Alzheimer’s disease. As part of the Graduate School for Systemic Neurosciences, my PhD project now focuses on the mechanisms linking amyloid-beta and tau pathology in early stages of AD. Outside the lab, I enjoy meeting friends, doing sports and trying to care for a growing collection of indoor plants.

Melina Paulus, Master’s student, Research Assistant
I obtained my Bachelor’s degree in Psychological and Brain Sciences from the University of California, Santa Barbara (2023) and went on to pursue a Master’s degree in Neuro-cognitive Psychology at LMU Munich. I joined Nico Franzmeiers lab in February 2024 as a research assistant, where I contribute to study coordination at the ISD, database management, and the evaluation of MRI imaging data. My first project explored the impacts of cerebral small vessel disease (SVD) on cognitive impairment in Alzheimer’s disease under the supervision of Anna Dewenter. I recently completed my Master’s thesis, which investigated the prognostic relevance of non-hemorrhagic MRI markers for Cerebral Amyloid Angiopathy Progression in Preclinical Alzheimer’s Disease. In my free time, I enjoy playing volleyball, pilates, and spending time with friends.
Alumni
Amir Dehsarvi, PhD, Postdoc
Julia Pescoller, Master’s student
Mattes Groß, MD student
Fabian Wagner, MD student
2024
2023
2022
2021
2020
2019
2018
2017
We gratefully acknowledge funding by the following funding agencies:

Thiemann Stiftung

Parkinson-Gesellschaft

Schick-Stiftung
Network of Centres of Excellence in Neurodegeneration (COEN)
COEN

Alzheimer’s Association

LMUexcellent
Die Alzheimer Forschung Initiative e.V.

Bright-Focus Foundation

Gemeinnützige Hertie Stiftung

Excellence program for research and funding (FöFoLe, LMU)

Lilly
Dr. Nicolai Franzmeier
Tel: +49 89 4400 46162
nicolai.franzmeier@med.uni-muenchen.de



